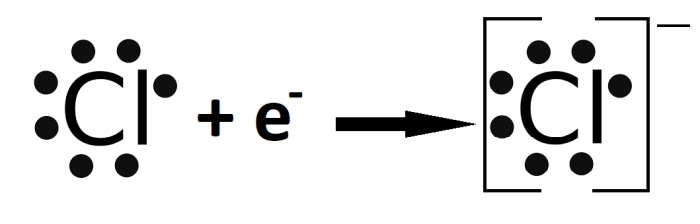Naming molecular compounds chem worksheet 9-2 takes center stage in this comprehensive guide, providing a systematic approach to deciphering the intricate language of chemistry. With precision and clarity, we embark on a journey to unravel the secrets of chemical nomenclature, empowering you to navigate the complexities of molecular identification.
Delving into the heart of the IUPAC system, we will explore the fundamental principles that govern the naming of molecular compounds. Prefixes and suffixes, the building blocks of chemical nomenclature, will be meticulously examined, revealing their significance in conveying vital information about atomic composition and bonding characteristics.
Chemical Nomenclature: Naming Molecular Compounds Chem Worksheet 9-2

Chemical nomenclature is a system of naming chemical compounds in a consistent and unambiguous manner. The International Union of Pure and Applied Chemistry (IUPAC) has developed a set of rules for naming molecular compounds, which are compounds composed of two or more nonmetal atoms.
The IUPAC system for naming molecular compounds is based on the following principles:
- The name of the compound is based on the names of the elements present in the compound.
- The prefixes mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca- are used to indicate the number of atoms of each element in the compound.
- The suffix -ide is used to indicate that the compound is an anion.
- The suffix -ate is used to indicate that the compound is an oxyanion.
For example, the compound NaCl is named sodium chloride. The name indicates that the compound is composed of one sodium atom and one chlorine atom.
Prefixes and Suffixes
The prefixes and suffixes used in IUPAC nomenclature are listed in the following table:
| Prefix | Number of atoms |
|---|---|
| mono- | 1 |
| di- | 2 |
| tri- | 3 |
| tetra- | 4 |
| penta- | 5 |
| hexa- | 6 |
| hepta- | 7 |
| octa- | 8 |
| nona- | 9 |
| deca- | 10 |
The suffixes -ide and -ate are used to indicate the type of bond between atoms.
- The suffix -ide is used to indicate that the compound is an anion.
- The suffix -ate is used to indicate that the compound is an oxyanion.
Naming Binary Molecular Compounds
Binary molecular compounds are compounds composed of two nonmetal elements. The steps for naming binary molecular compounds are as follows:
- Write the name of the first element in the compound.
- Add the suffix
ide to the name of the second element.
- Use the prefixes mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca- to indicate the number of atoms of each element in the compound.
For example, the compound CO is named carbon monoxide. The name indicates that the compound is composed of one carbon atom and one oxygen atom.
Naming Acids
Acids are compounds that contain hydrogen atoms that can be donated to other compounds. The IUPAC system for naming acids is as follows:
- Write the name of the element in the acid.
- Add the suffix
ic to the name of the element.
- If the acid contains more than one hydrogen atom, use the prefixes di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca- to indicate the number of hydrogen atoms in the acid.
For example, the acid HCl is named hydrochloric acid. The name indicates that the acid contains one hydrogen atom and one chlorine atom.
Naming Salts, Naming molecular compounds chem worksheet 9-2
Salts are compounds composed of a metal cation and a nonmetal anion. The IUPAC system for naming salts is as follows:
- Write the name of the metal cation.
- Add the name of the nonmetal anion.
For example, the salt NaCl is named sodium chloride. The name indicates that the salt is composed of a sodium cation and a chloride anion.
Naming Organic Compounds
Organic compounds are compounds that contain carbon atoms. The IUPAC system for naming organic compounds is based on the following principles:
- The name of the compound is based on the name of the parent hydrocarbon.
- The prefixes mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca- are used to indicate the number of carbon atoms in the parent hydrocarbon.
- The suffixes -ane, -ene, and -yne are used to indicate the type of bond between the carbon atoms in the parent hydrocarbon.
For example, the compound CH 4is named methane. The name indicates that the compound is composed of one carbon atom and four hydrogen atoms.
FAQ Overview
What is the significance of using prefixes in chemical nomenclature?
Prefixes play a crucial role in indicating the number of atoms of a particular element present in a molecular compound.
How do suffixes contribute to naming molecular compounds?
Suffixes provide essential information about the type of bond between atoms, distinguishing between single, double, and triple bonds.
What is the purpose of parentheses in naming polyatomic ions?
Parentheses are used to group polyatomic ions within a compound, ensuring clarity and preventing confusion in naming.